Decoding the Physique’s pH: A Complete Information to Understanding and Sustaining Acid-Base Stability
Associated Articles: Decoding the Physique’s pH: A Complete Information to Understanding and Sustaining Acid-Base Stability
Introduction
With nice pleasure, we’ll discover the intriguing matter associated to Decoding the Physique’s pH: A Complete Information to Understanding and Sustaining Acid-Base Stability. Let’s weave attention-grabbing info and provide recent views to the readers.
Desk of Content material
Decoding the Physique’s pH: A Complete Information to Understanding and Sustaining Acid-Base Stability
The human physique is a marvel of intricate biochemical processes, continuously striving for homeostasis – a state of inner equilibrium. A vital facet of this equilibrium is sustaining the proper pH stage, a measure of acidity or alkalinity. Whereas typically mentioned within the context of dietary developments and different medication, understanding the physique’s pH ranges is crucial for appreciating its advanced physiology and recognizing potential well being points. This text delves into the intricacies of the physique’s pH, exploring its variations throughout completely different programs, the mechanisms that regulate it, and the implications of imbalances.
What’s pH and Why Does it Matter?
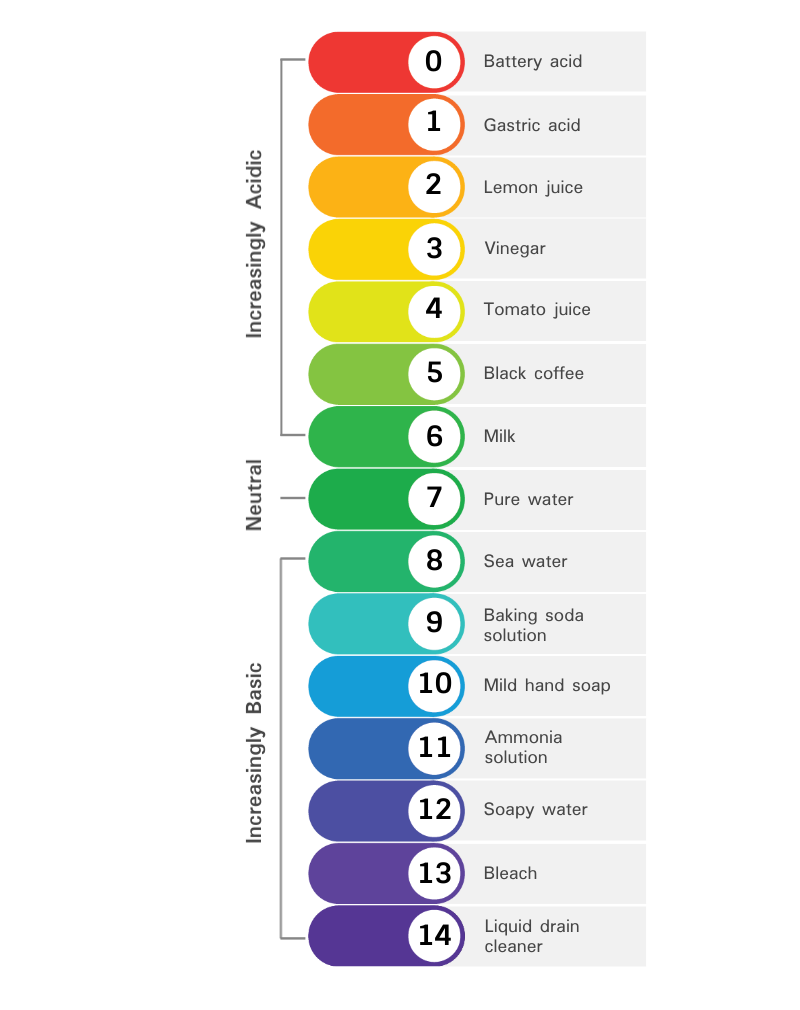
pH is a logarithmic scale that measures the focus of hydrogen ions (H+) in an answer. A pH of seven is impartial, with values beneath 7 indicating acidity and values above 7 indicating alkalinity. Every entire quantity change on the pH scale represents a tenfold change in H+ focus. As an illustration, a pH of 6 is ten occasions extra acidic than a pH of seven.
Sustaining a exact pH is significant for quite a few bodily capabilities. Enzymes, the organic catalysts that drive metabolic reactions, are extremely delicate to pH modifications. Even slight deviations from the optimum pH can considerably impair their exercise, disrupting essential metabolic pathways. Moreover, pH influences the construction and performance of proteins, affecting mobile processes and total physiological integrity. Electrolyte stability, nerve impulse transmission, and muscle contraction are all profoundly influenced by the physique’s pH.
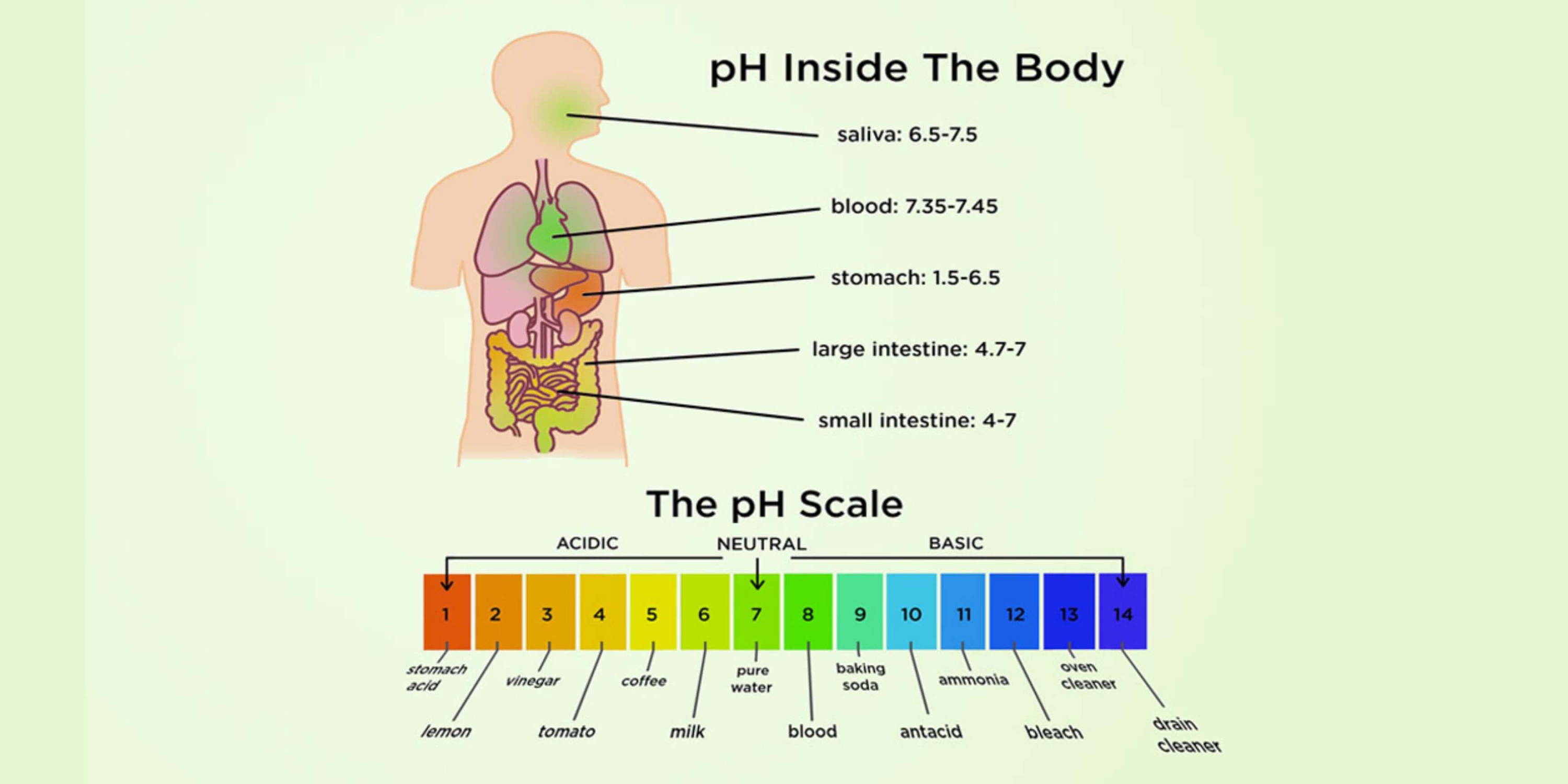
pH Ranges in Totally different Physique Methods: A Chart Overview
It is essential to grasp that the physique is not uniformly acidic or alkaline. Totally different bodily fluids and compartments preserve distinct pH ranges, every optimized for particular capabilities. The next chart supplies a normal overview:
| Physique Fluid/Compartment | Regular pH Vary | Significance of Deviation |
|---|---|---|
| Blood (Arterial) | 7.35 – 7.45 | Acidosis (beneath 7.35): Respiratory or metabolic; Alkalosis (above 7.45): Respiratory or metabolic |
| Blood (Venous) | 7.32 – 7.42 | Barely extra acidic than arterial blood resulting from CO2 accumulation |
| Cerebrospinal Fluid | 7.32 – 7.34 | Just like venous blood; essential for mind perform |
| Gastric Juice | 1.5 – 3.5 | Extremely acidic; important for protein digestion |
| Pancreatic Juice | 7.5 – 8.0 | Alkaline; neutralizes acidic chyme getting into the duodenum |
| Intestinal Fluid | 7.0 – 8.0 | Varies alongside the intestinal tract; influences nutrient absorption |
| Urine | 4.6 – 8.0 | Extremely variable relying on weight loss program and metabolic state; displays kidney’s position in acid-base stability |
| Saliva | 6.2 – 7.4 | Barely acidic to impartial; aids in oral hygiene and digestion |
| Vaginal Fluid | 3.8 – 4.5 | Barely acidic; protects towards an infection |
Mechanisms of pH Regulation: The Physique’s Protection System
The human physique employs a number of subtle mechanisms to take care of its exact pH ranges. These embrace:
-
Buffer Methods: These act as chemical sponges, absorbing extra H+ or OH- ions to stop drastic pH modifications. The bicarbonate buffer system, involving carbonic acid (H2CO3) and bicarbonate ions (HCO3-), is the first buffer in blood. Phosphate and protein buffers additionally play important roles.
-
Respiratory System: The lungs play an important position in regulating blood pH by controlling the extent of carbon dioxide (CO2). CO2 dissolves in blood to type carbonic acid, which might dissociate into H+ and bicarbonate ions. Elevated respiration (hyperventilation) expels CO2, lowering H+ focus and rising pH (alkalosis). Conversely, decreased respiration (hypoventilation) retains CO2, rising H+ focus and reducing pH (acidosis).
-
Renal System: The kidneys are the physique’s final pH regulators. They selectively reabsorb or excrete bicarbonate ions and H+ ions, fine-tuning the blood pH to take care of its optimum vary. Additionally they excrete acids like uric acid and phosphate, additional contributing to acid-base stability.
Penalties of pH Imbalance: Acidosis and Alkalosis
Deviations from the traditional pH ranges can result in severe well being penalties.
-
Acidosis (low pH): This could outcome from numerous causes, together with respiratory issues (e.g., pneumonia, emphysema), metabolic issues (e.g., diabetes, kidney failure), or extreme acid consumption. Signs can vary from fatigue and complications to nausea, vomiting, and even coma.
-
Alkalosis (excessive pH): This may be brought on by extreme vomiting, extreme consumption of antacids, or respiratory circumstances like hyperventilation. Signs embrace muscle weak point, tremors, tingling sensations, and confusion.
The Position of Eating regimen and Life-style in Sustaining pH Stability
Whereas the physique’s regulatory mechanisms are extremely efficient, weight loss program and life-style selections can affect pH stability. The notion of "alkaline diets," emphasizing vegetables and fruit, goals to cut back acidity. Nevertheless, the influence of weight loss program on total physique pH is advanced and infrequently oversimplified. Whereas the weight loss program can have an effect on the pH of urine, its affect on blood pH is restricted because of the physique’s sturdy buffering programs. Nevertheless, a balanced weight loss program wealthy in fruits, greens, and entire grains supplies important vitamins and antioxidants that assist total well being, not directly contributing to higher pH regulation. Hydration can also be essential, as adequate water consumption helps kidney perform and helps remove waste merchandise that contribute to acidity.
Medical Evaluation of pH Imbalance
Diagnosing acid-base imbalances entails measuring blood pH and analyzing blood fuel ranges (partial pressures of oxygen and carbon dioxide), in addition to bicarbonate ranges. Urine pH may present invaluable info. The underlying explanation for the imbalance have to be recognized and addressed by acceptable medical intervention. This would possibly contain treating the underlying illness, administering fluids, or offering respiratory assist.
Conclusion:
Sustaining the physique’s intricate pH stability is crucial for optimum well being and well-being. Whereas the physique possesses highly effective mechanisms to manage pH, understanding the elements that may affect it and recognizing the indicators of imbalance are essential. A wholesome life-style, together with a balanced weight loss program, ample hydration, and common train, helps the physique’s pure capability to take care of its delicate acid-base equilibrium. Nevertheless, important deviations from regular pH ranges warrant fast medical consideration, as they will point out severe underlying well being circumstances. This text supplies a complete overview, however it isn’t an alternative choice to skilled medical recommendation. Seek the advice of a healthcare supplier for any issues relating to your physique’s pH or acid-base stability.







Closure
Thus, we hope this text has supplied invaluable insights into Decoding the Physique’s pH: A Complete Information to Understanding and Sustaining Acid-Base Stability. We thanks for taking the time to learn this text. See you in our subsequent article!