Decoding the Chart of Nuclides: A Journey By means of the Panorama of Atomic Nuclei
Associated Articles: Decoding the Chart of Nuclides: A Journey By means of the Panorama of Atomic Nuclei
Introduction
With nice pleasure, we are going to discover the intriguing subject associated to Decoding the Chart of Nuclides: A Journey By means of the Panorama of Atomic Nuclei. Let’s weave attention-grabbing info and supply recent views to the readers.
Desk of Content material
Decoding the Chart of Nuclides: A Journey By means of the Panorama of Atomic Nuclei
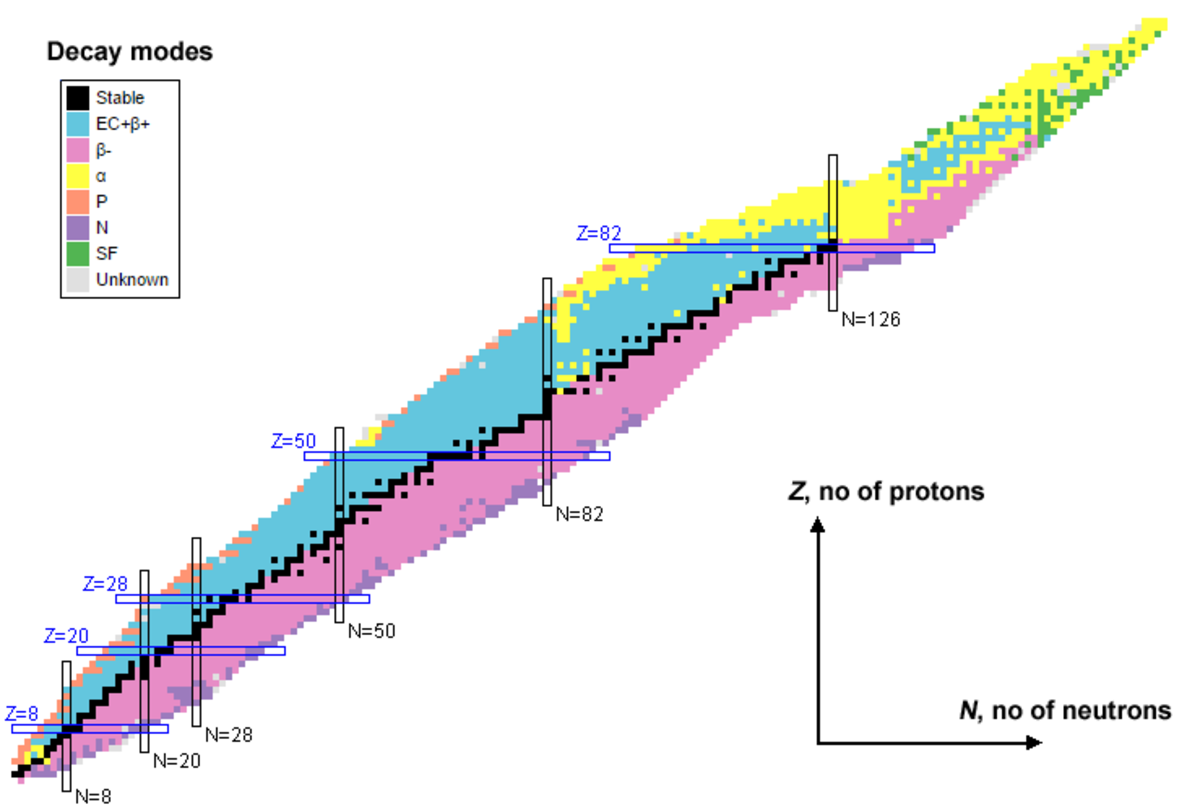
The Chart of Nuclides, a seemingly advanced grid of numbers and symbols, is in actuality a robust visible illustration of the identified isotopes of all components. It is a elementary instrument in nuclear physics, nuclear medication, nuclear engineering, and numerous different scientific disciplines, offering a wealth of details about the soundness and properties of atomic nuclei. This text will discover the construction, interpretation, and purposes of the Chart of Nuclides, delving into its significance for our understanding of the atomic world.
Understanding the Fundamentals: Isotopes and Nuclides
Earlier than diving into the intricacies of the chart, it is essential to outline some key phrases. An isotope refers to atoms of the identical factor (i.e., possessing the identical variety of protons) however with differing numbers of neutrons. The variety of protons determines the factor’s atomic quantity (Z), whereas the sum of protons and neutrons defines the mass quantity (A). A nuclide is a particular atomic nucleus characterised by its distinctive mixture of protons and neutrons – basically, a particular isotope. The Chart of Nuclides meticulously organizes all identified nuclides, providing a complete overview of their properties.
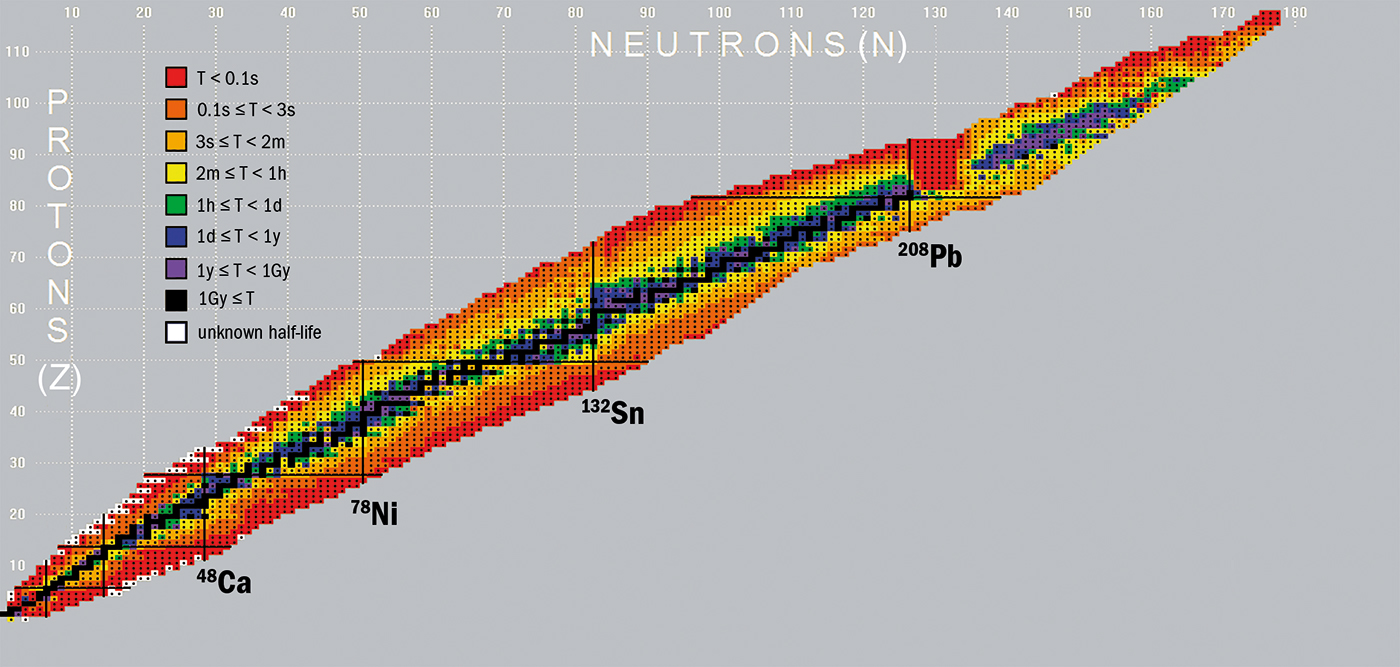
The Construction of the Chart of Nuclides
The Chart of Nuclides sometimes shows nuclides organized in a grid format. The horizontal axis normally represents the neutron quantity (N), whereas the vertical axis represents the proton quantity (Z). Every sq. within the grid represents a particular nuclide, recognized by its image (e.g., ¹²C for carbon-12) and infrequently accompanied by further knowledge. This knowledge can embrace:
- Mass quantity (A): The entire variety of protons and neutrons within the nucleus.
- Half-life (t½): The time it takes for half of a given quantity of a radioactive isotope to decay. That is essential for understanding the soundness and decay traits of nuclides. Half-lives vary from fractions of a second to billions of years.
- Decay modes: The methods through which a radioactive nuclide decays, reminiscent of alpha decay (emission of an alpha particle), beta decay (emission of a beta particle), gamma decay (emission of a gamma ray), or spontaneous fission. These decay modes are sometimes represented by arrows indicating the daughter nuclide produced.
- Abundance: For steady nuclides, this means their pure abundance on Earth.
- Spin and parity: Quantum numbers describing the intrinsic angular momentum and parity of the nucleus.
- Neutron seize cross-section: The chance of a nucleus absorbing a neutron. That is particularly vital in nuclear reactor design and neutron activation evaluation.
- Power ranges: For some nuclides, vitality ranges of excited nuclear states could also be proven.
Navigating the Chart: Stability and Instability
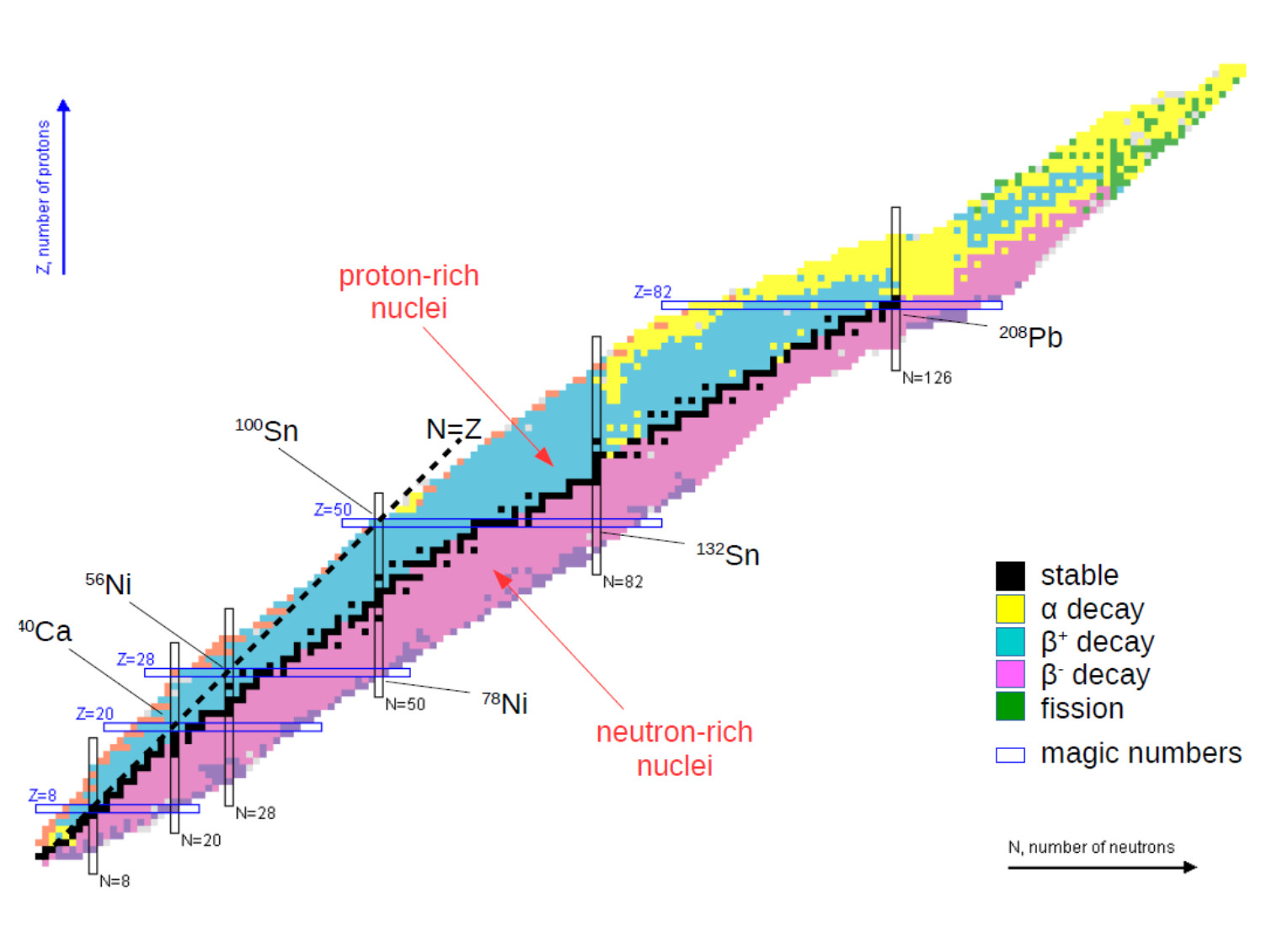
The Chart of Nuclides reveals fascinating patterns concerning nuclear stability. The "valley of stability" is a outstanding function, representing the area the place nuclides are comparatively steady. Nuclides removed from this valley are typically extremely radioactive and decay quickly. A number of tendencies are observable:
- Neutron-to-proton ratio (N/Z): For lighter components, a steady N/Z ratio is near 1. Because the atomic quantity will increase, the optimum N/Z ratio step by step will increase to take care of stability. Nuclides with N/Z ratios considerably deviating from this optimum are sometimes unstable.
- Magic numbers: Sure neutron and proton numbers (2, 8, 20, 28, 50, 82, 126) are related to enhanced nuclear stability. Nuclides with these "magic numbers" of protons or neutrons are sometimes extra steady than their neighbors. These numbers mirror the filling of nuclear shells, analogous to electron shells in atoms.
- Isobaric traces: Traces of fixed mass quantity (A) characterize isobars – nuclides with the identical mass quantity however completely different numbers of protons and neutrons. Isobaric traces usually present a collection of radioactive decays resulting in a steady isobar.
- Isotonic traces: Traces of fixed neutron quantity (N) characterize isotones – nuclides with the identical neutron quantity however completely different numbers of protons. Analyzing isotonic traces can present insights into the position of neutrons in nuclear stability.
Purposes of the Chart of Nuclides
The Chart of Nuclides isn’t merely a theoretical assemble; it has quite a few sensible purposes throughout numerous fields:
- Nuclear medication: The chart is indispensable for choosing acceptable radioisotopes for diagnostic and therapeutic functions. The half-life, decay mode, and vitality of emitted radiation are essential components in selecting appropriate radiotracers for imaging strategies like PET (positron emission tomography) and SPECT (single-photon emission computed tomography), or for radiotherapy.
- Nuclear engineering: The chart is crucial for designing and working nuclear reactors. Understanding neutron seize cross-sections and decay traits of assorted nuclides is important for reactor security, gas administration, and waste disposal.
- Nuclear forensics: The chart aids in figuring out the origin and nature of nuclear supplies in investigations associated to nuclear proliferation and safety. The isotopic composition of supplies can present clues about their supply and processing historical past.
- Geochronology: Sure radioactive isotopes with lengthy half-lives, reminiscent of uranium and potassium isotopes, are utilized in radiometric courting to find out the age of geological formations and artifacts. The decay chains of those isotopes are clearly depicted on the chart.
- Analysis and training: The Chart of Nuclides serves as a elementary instrument in nuclear physics analysis, offering a complete database of nuclear properties for theoretical modeling and experimental evaluation. It is also a invaluable instructional useful resource for college students studying about nuclear construction and radioactivity.
Past the Fundamental Chart: Increasing Our Information
Whereas the fundamental Chart of Nuclides supplies a complete overview of identified nuclides, ongoing analysis continues to develop our understanding of the nuclear panorama. This consists of:
- The seek for new isotopes: Scientists are always trying to find new, usually extremely unstable, isotopes, pushing the boundaries of the identified nuclide chart. These discoveries usually problem our theoretical fashions of nuclear construction and stability.
- Detailed nuclear knowledge: Superior charts incorporate extra detailed details about nuclear properties, reminiscent of excited nuclear states, decay branching ratios, and nuclear moments.
- On-line interactive charts: The arrival of digital expertise has led to the event of interactive on-line variations of the Chart of Nuclides, providing enhanced search capabilities, visualization instruments, and entry to complete databases.
Conclusion
The Chart of Nuclides is a outstanding instrument that encapsulates an enormous quantity of details about the atomic nucleus. Its visible illustration of isotopes and their properties supplies a elementary understanding of nuclear stability, decay processes, and the conduct of atomic nuclei. Its purposes span numerous fields, from nuclear medication and engineering to geological courting and forensic science. As analysis continues to develop our data of the nuclear world, the Chart of Nuclides will stay an indispensable useful resource, guiding our exploration of this fascinating and sophisticated realm of matter. Its continued growth and accessibility make sure that this highly effective instrument will stay a cornerstone of nuclear science for years to return.





Closure
Thus, we hope this text has supplied invaluable insights into Decoding the Chart of Nuclides: A Journey By means of the Panorama of Atomic Nuclei. We hope you discover this text informative and helpful. See you in our subsequent article!