Decoding the Dance of Atoms: Understanding and Using the Reactivity Chart of the Components
Associated Articles: Decoding the Dance of Atoms: Understanding and Using the Reactivity Chart of the Components
Introduction
On this auspicious event, we’re delighted to delve into the intriguing matter associated to Decoding the Dance of Atoms: Understanding and Using the Reactivity Chart of the Components. Let’s weave attention-grabbing data and supply recent views to the readers.
Desk of Content material
Decoding the Dance of Atoms: Understanding and Using the Reactivity Chart of the Components

The periodic desk, a cornerstone of chemistry, organizes parts primarily based on their atomic construction and recurring properties. Nonetheless, understanding how these parts work together – their reactivity – requires a deeper dive. Whereas the periodic desk supplies clues, a reactivity chart affords a extra visible and intuitive understanding of the chemical conduct of parts. This chart does not exist as a single, universally accepted customary just like the periodic desk, however reasonably takes varied kinds relying on the precise focus (e.g., reactivity with water, acids, oxygen). This text will discover the basic rules behind elemental reactivity, look at the widespread options of varied reactivity charts, and talk about their purposes in various fields.
The Basis of Reactivity: Electron Configuration and Electronegativity
A component’s reactivity is primarily decided by its electron configuration, particularly the variety of valence electrons – the electrons within the outermost shell. Atoms try for stability, typically achieved by having a full outermost shell (eight electrons, aside from hydrogen and helium, which require two). This drive for stability dictates how readily a component will acquire, lose, or share electrons with different atoms, resulting in chemical reactions.
Electronegativity, a measure of an atom’s skill to draw electrons in a chemical bond, performs a vital function. Extremely electronegative parts strongly entice electrons, making them extra more likely to acquire electrons and type unfavorable ions (anions). Conversely, parts with low electronegativity readily lose electrons, forming optimistic ions (cations). The distinction in electronegativity between two atoms determines the kind of bond they may type: ionic (giant distinction), covalent (small distinction), or metallic (between related atoms).
Widespread Themes in Reactivity Charts
Whereas particular reactivity charts fluctuate, a number of widespread themes emerge:
-
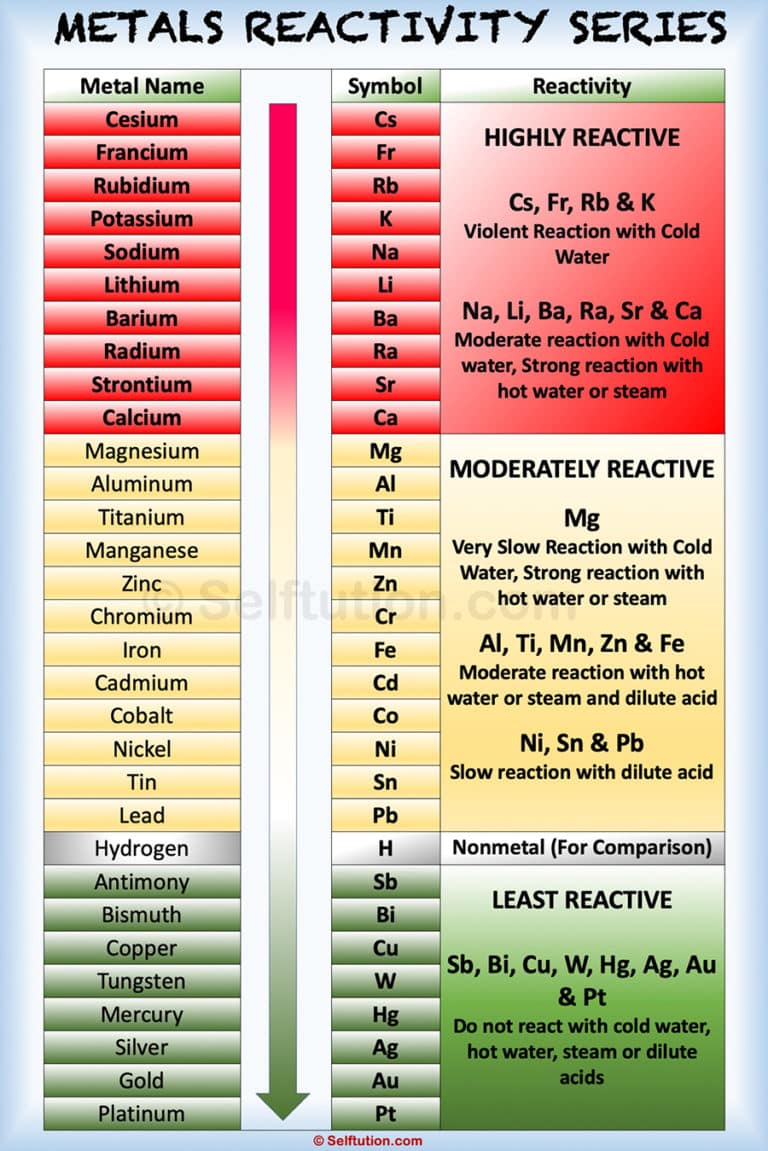
Group Developments: Components inside the identical group (vertical column) of the periodic desk typically exhibit related reactivity patterns. For example, alkali metals (Group 1) are extremely reactive as a result of they readily lose one electron to realize a secure configuration. Halogens (Group 17) are additionally extremely reactive, readily gaining one electron to finish their outermost shell. Noble gases (Group 18), with their full valence shells, are exceptionally unreactive.
-
Interval Developments: Throughout a interval (horizontal row), reactivity typically modifications. On the left aspect, metallic parts are typically extra reactive, shedding electrons simply. As you progress to the fitting, parts turn into much less metallic and extra electronegative, growing their tendency to achieve electrons. The transition metals (within the center) present extra complicated reactivity patterns because of the involvement of a number of electron shells in bonding.
-
Visible Illustration: Reactivity charts typically make use of color-coding or a scale to characterize the relative reactivity of parts. Extremely reactive parts could be represented by intense colours or excessive numerical values, whereas much less reactive parts are proven with paler colours or decrease values. This visible illustration permits for fast comparability and identification of reactive parts.
-
Particular Response Varieties: Many reactivity charts give attention to a selected sort of response, reminiscent of reactivity with water, acids, or oxygen. This specialization supplies a centered view of elemental conduct beneath particular situations. For instance, a chart illustrating reactivity with water would present the vigorous response of alkali metals contrasted with the inertness of noble gases.
Forms of Reactivity Charts and Their Functions
A number of sorts of reactivity charts cater to particular wants:
-
Metallic Reactivity Collection: This chart ranks metals primarily based on their reactivity with acids or water. Extremely reactive metals like potassium react violently with water, whereas much less reactive metals like copper don’t react in any respect. This collection is prime in predicting the end result of single displacement reactions and understanding the relative ease of steel extraction.
-
Non-metal Reactivity Collection: Just like the steel reactivity collection, this chart ranks non-metals primarily based on their reactivity, typically with different parts or compounds. For example, it would present the relative ease with which halogens react with different parts.
-
Electrochemical Collection: This chart lists parts primarily based on their customary discount potentials. It is essential in electrochemistry, predicting the spontaneity of redox reactions and the potential distinction in electrochemical cells. The collection helps decide which parts will act as oxidizing brokers (settle for electrons) and which can act as decreasing brokers (donate electrons).
-
Reactivity with Oxygen: This chart reveals the relative ease with which parts react with oxygen to type oxides. Some parts, like alkali metals, react quickly and vigorously with oxygen, whereas others react slowly or in no way. That is important for understanding combustion processes and the steadiness of supplies in oxygen-rich environments.
-
Reactivity with Water: This chart focuses on the reactions of parts with water, showcasing the various vary of reactions from explosive (alkali metals) to no response (noble gases). This chart is important in understanding the conduct of supplies in aqueous environments and predicting potential hazards.
Functions of Reactivity Charts
Reactivity charts have quite a few purposes throughout varied scientific and engineering disciplines:
-
Predicting Response Outcomes: Charts assist predict whether or not a response will happen and its probably consequence. For instance, a steel reactivity collection can predict whether or not a steel will displace one other steel from an answer.
-
Materials Choice: In engineering, reactivity charts are important for choosing acceptable supplies for particular purposes. For instance, selecting a corrosion-resistant steel for a marine setting requires contemplating its reactivity with seawater.
-
Chemical Synthesis: Charts assist in designing chemical synthesis pathways by predicting the reactivity of various reagents and choosing acceptable response situations.
-
Environmental Science: Understanding elemental reactivity is essential for assessing environmental impacts. For instance, the reactivity of pollution with water or soil can decide their destiny and transport within the setting.
-
Geochemistry: Reactivity charts assist perceive geological processes, reminiscent of mineral formation and weathering, primarily based on the interplay of parts in numerous environments.
Limitations and Issues
Whereas reactivity charts are invaluable instruments, it is essential to acknowledge their limitations:
-
Simplified Illustration: Charts typically simplify complicated interactions, neglecting components like temperature, strain, focus, and the presence of catalysts. Actual-world reactions are not often as simple as depicted in a chart.
-
Relative Reactivity: Charts typically present relative reactivity reasonably than absolute values. The precise response price or extent is determined by quite a few components past what the chart depicts.
-
Dynamic Nature: Reactivity might be influenced by varied components, together with the bodily state of the component (strong, liquid, gasoline) and the presence of different substances. A chart won’t seize all these nuances.
Conclusion
Reactivity charts present a strong visible instrument for understanding and predicting the chemical conduct of parts. Whereas they provide a simplified illustration of complicated interactions, they’re indispensable for varied purposes throughout science and engineering. By understanding the underlying rules of electron configuration and electronegativity, and by critically evaluating the restrictions of those charts, we are able to successfully make the most of them to interpret chemical conduct and make knowledgeable choices in various fields. As our understanding of chemical interactions deepens, so too will the sophistication and accuracy of those important instruments.





Closure
Thus, we hope this text has supplied useful insights into Decoding the Dance of Atoms: Understanding and Using the Reactivity Chart of the Components. We hope you discover this text informative and useful. See you in our subsequent article!