Decoding the Solubility Chart: A Complete Information
Associated Articles: Decoding the Solubility Chart: A Complete Information
Introduction
On this auspicious event, we’re delighted to delve into the intriguing matter associated to Decoding the Solubility Chart: A Complete Information. Let’s weave attention-grabbing info and supply contemporary views to the readers.
Desk of Content material
Decoding the Solubility Chart: A Complete Information

Solubility charts are important instruments in chemistry, offering a visible illustration of the solubility of assorted substances in numerous solvents at various temperatures. Understanding easy methods to interpret these charts is essential for a variety of functions, from predicting response outcomes in chemical synthesis to designing efficient separation methods in analytical chemistry. This text gives a complete information to studying and understanding solubility charts, masking their development, interpretation, and sensible functions.
I. Understanding the Fundamentals of Solubility
Earlier than diving into solubility charts, it is necessary to understand the elemental idea of solubility. Solubility refers back to the most quantity of a solute (the substance being dissolved) that may dissolve in a given quantity of solvent (the substance doing the dissolving) at a particular temperature and stress. This most quantity is usually expressed as grams of solute per 100 grams of solvent (g/100g) or as molarity (moles of solute per liter of answer, mol/L). The solubility of a substance is influenced by a number of elements:
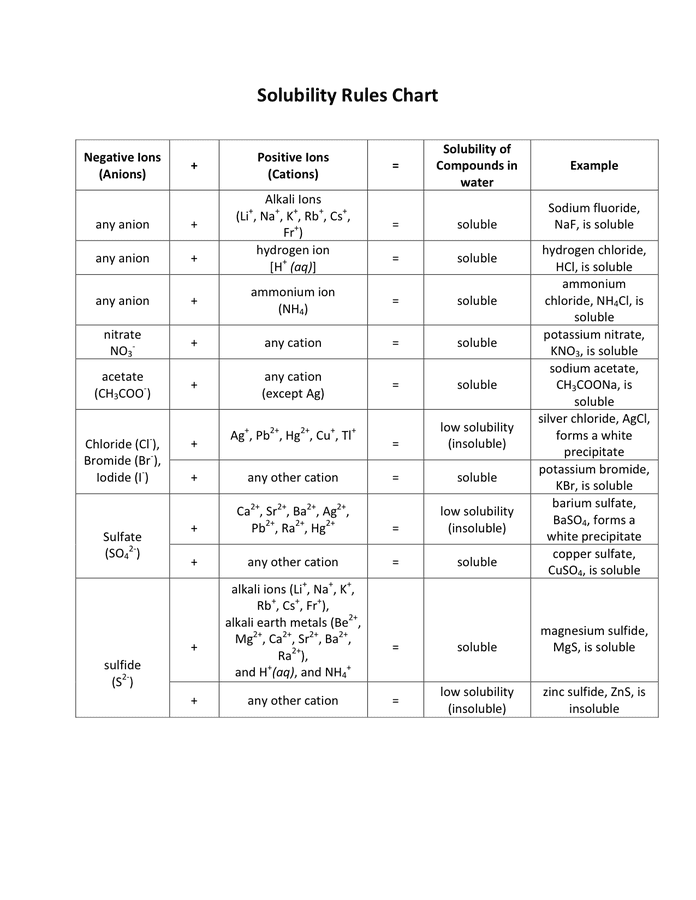
- Nature of the solute and solvent: "Like dissolves like" is a elementary precept. Polar solvents (e.g., water) are inclined to dissolve polar solutes (e.g., salts, sugars), whereas nonpolar solvents (e.g., hexane) dissolve nonpolar solutes (e.g., oils, fat).
- Temperature: The solubility of most solids in liquids will increase with rising temperature. Nevertheless, the solubility of gases in liquids usually decreases with rising temperature.
- Strain: Strain considerably impacts the solubility of gases in liquids. Henry’s Regulation states that the solubility of a gasoline is immediately proportional to the partial stress of that gasoline above the liquid. The impact of stress on the solubility of solids is negligible.
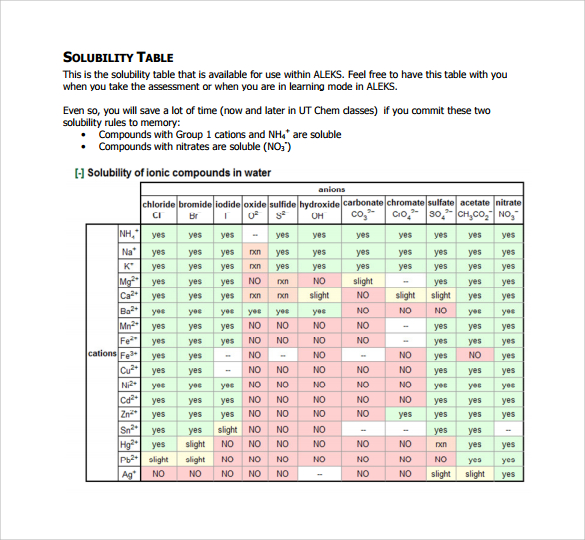
II. Construction and Interpretation of Solubility Charts
Solubility charts usually current solubility information graphically. The commonest sort makes use of a two-dimensional plot, with temperature on the x-axis and solubility (normally in g/100g) on the y-axis. Every line or curve on the chart represents the solubility of a particular substance in a particular solvent.
A. Figuring out Parts:
-
Axes Labels: Fastidiously study the axes labels to grasp the models used for temperature (normally in °C or °F) and solubility (g/100g, mol/L, or different models). Incorrectly deciphering the models can result in vital errors.
-
Substance Identification: The chart will clearly determine the solute and solvent. For instance, a chart would possibly present the solubility of potassium nitrate (KNO₃) in water. A number of solutes could also be represented on a single chart, every with its personal line or curve.
-
Solubility Curves: Every curve represents the solubility of a particular substance at totally different temperatures. The curve’s place signifies the solubility at a given temperature. A steeper curve signifies a better change in solubility with temperature. A flat curve signifies minimal change in solubility over the temperature vary.
B. Extracting Info:
-
Discovering Solubility at a Particular Temperature: To find out the solubility of a substance at a selected temperature, discover the temperature on the x-axis and hint a vertical line upwards till it intersects the solubility curve for that substance. Then, hint a horizontal line from the intersection level to the y-axis to learn the solubility worth.
-
Figuring out Temperature for a Given Solubility: Conversely, if the specified solubility, discover that worth on the y-axis and hint a horizontal line to the proper till it intersects the solubility curve. Then, hint a vertical line right down to the x-axis to search out the corresponding temperature.
-
Evaluating Solubilities: Solubility charts permit for straightforward comparability of the solubilities of various substances on the similar temperature or the solubility of a single substance at totally different temperatures. That is essential for selecting acceptable solvents and predicting the outcomes of crystallization or precipitation processes.
C. Particular Concerns:
-
Supersaturation: It is necessary to do not forget that solubility charts characterize the most solubility. Options can exist in a supersaturated state, the place the focus of solute exceeds its equilibrium solubility. These options are unstable and can finally precipitate extra solute.
-
Items Consistency: Guarantee consistency in models when performing calculations or comparisons. Convert all values to the identical models earlier than making any calculations.
-
Temperature Vary: Take note of the temperature vary lined by the chart. Extrapolating past this vary can result in inaccurate predictions.
III. Purposes of Solubility Charts
Solubility charts are invaluable instruments in numerous chemical and engineering functions:
-
Recrystallization: In natural chemistry, recrystallization is a standard purification method. Solubility charts assist decide the optimum solvent and temperature circumstances for dissolving the impure compound after which selectively crystallizing it out because it cools, leaving impurities behind in answer.
-
Precipitation Reactions: Solubility charts are used to foretell whether or not a precipitate will type when two options are combined. If the focus of a product exceeds its solubility at a given temperature, a precipitate will type.
-
Separation Strategies: Strategies like fractional crystallization depend on variations in solubility to separate mixtures of gear. Solubility charts information the collection of acceptable solvents and temperatures for efficient separation.
-
Environmental Science: Solubility information is essential for understanding the destiny of pollution within the setting. Solubility charts assist predict the transport and bioavailability of contaminants in water and soil.
-
Pharmaceutical Trade: Solubility is a important consider drug formulation and supply. Solubility charts are used to find out the optimum circumstances for dissolving and delivering medication within the physique.
-
Geological Sciences: Understanding mineral solubility is crucial for deciphering geological processes. Solubility charts assist predict mineral formation and dissolution underneath various circumstances.
IV. Past Easy Solubility Charts
Whereas the essential two-dimensional solubility chart is extensively used, extra complicated representations exist. These could embody:
-
Three-dimensional charts: These charts add one other dimension, usually representing stress or focus of one other element within the system.
-
Solubility diagrams: These diagrams present the solubility areas of various phases (stable, liquid, gasoline) as a operate of temperature and composition.
-
Pc simulations: Superior software program packages can mannequin solubility conduct underneath complicated circumstances, offering extra correct and detailed predictions than easy charts.
V. Conclusion
Solubility charts are elementary instruments for understanding and predicting the conduct of options. By understanding the essential ideas of solubility and the construction and interpretation of solubility charts, chemists, engineers, and scientists can successfully make the most of this info in a wide range of functions. Mastering the flexibility to learn and interpret these charts is an important ability for anybody working in fields that contain options and chemical reactions. Keep in mind to all the time fastidiously study the axes labels, models, and the precise substances concerned to keep away from misinterpretations and guarantee correct outcomes. With cautious consideration to element, solubility charts generally is a highly effective device in fixing a big selection of chemical issues.





Closure
Thus, we hope this text has supplied helpful insights into Decoding the Solubility Chart: A Complete Information. We respect your consideration to our article. See you in our subsequent article!