Decoding the Universe: A Deep Dive into the Electron Configuration Chart of the Parts
Associated Articles: Decoding the Universe: A Deep Dive into the Electron Configuration Chart of the Parts
Introduction
With enthusiasm, let’s navigate by way of the intriguing subject associated to Decoding the Universe: A Deep Dive into the Electron Configuration Chart of the Parts. Let’s weave fascinating info and provide contemporary views to the readers.
Desk of Content material
Decoding the Universe: A Deep Dive into the Electron Configuration Chart of the Parts
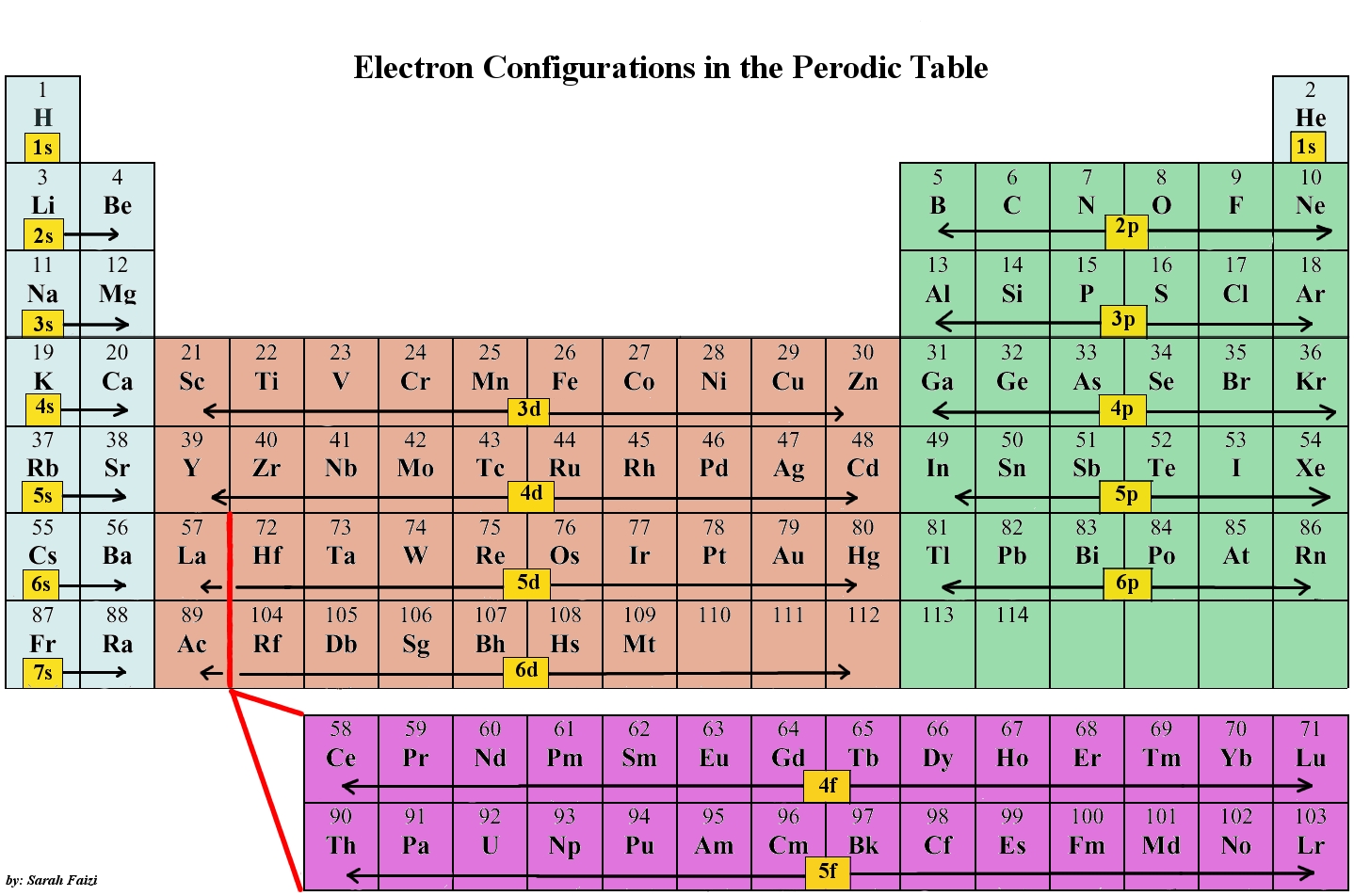
The periodic desk, a seemingly easy association of components, holds inside it the secrets and techniques of atomic construction and chemical habits. On the coronary heart of this understanding lies the electron configuration, an outline of how electrons are organized throughout the power ranges and sublevels of an atom. This text will discover the electron configuration chart, inspecting its rules, patterns, exceptions, and its essential function in predicting the properties of components.
Understanding the Fundamentals: Vitality Ranges, Sublevels, and Orbitals
Earlier than diving into the electron configuration chart itself, we have to grasp basic ideas. Electrons, negatively charged particles, reside in power ranges surrounding the atom’s nucleus. These ranges will not be steady; they’re quantized, which means electrons can solely exist at particular power ranges. These ranges are numbered, with stage 1 being closest to the nucleus and having the bottom power.
Every power stage is additional divided into sublevels, designated by the letters s, p, d, and f. These sublevels symbolize totally different shapes and orientations of atomic orbitals. Orbitals are areas of house the place there is a excessive likelihood of discovering an electron.
- s sublevel: Holds a most of two electrons and has a spherical form.
- p sublevel: Holds a most of 6 electrons and has a dumbbell form with three mutually perpendicular orientations (px, py, pz).
- d sublevel: Holds a most of 10 electrons and has extra advanced shapes.
- f sublevel: Holds a most of 14 electrons and possesses much more intricate shapes.
The order of filling these sublevels follows the Aufbau precept, which states that electrons first fill the bottom power ranges obtainable. Nevertheless, the power ranges aren’t all the time completely sequential; the 4s sublevel, for instance, fills earlier than the 3d sublevel. That is mirrored within the electron configuration chart.
The Electron Configuration Chart: A Visible Illustration
The electron configuration chart, typically introduced as a desk or diagram, systematically exhibits the electron configuration for every factor. It sometimes lists the factor’s image, atomic quantity (variety of protons and electrons), and the electron association within the type of sublevel notation. For instance, the electron configuration of hydrogen (atomic #1) is 1s¹, indicating one electron within the 1s sublevel. Helium (atomic quantity 2) is 1s².
The chart reveals a transparent sample, significantly inside intervals (horizontal rows) and teams (vertical columns) of the periodic desk. Parts throughout the similar group share comparable outermost electron configurations, resulting in comparable chemical properties. As an illustration, the alkali metals (Group 1) all have a single electron of their outermost s sublevel (ns¹), explaining their excessive reactivity.
Constructing the Chart: Step-by-Step Instance
Let’s assemble the electron configuration for just a few components for example the method:
-
Sodium (Na, atomic quantity 11): Following the Aufbau precept, we fill the sublevels so as of accelerating power: 1s², 2s², 2p⁶, 3s¹. This exhibits two electrons within the first power stage, eight within the second, and one within the third. The outermost electron (3s¹) is liable for sodium’s reactivity.
-
Chlorine (Cl, atomic quantity 17): Its configuration is 1s², 2s², 2p⁶, 3s², 3p⁵. The seven electrons within the outermost shell (3s²3p⁵) clarify chlorine’s tendency to realize one electron to attain a steady octet.
-
Iron (Fe, atomic quantity 26): This presents a barely extra advanced situation: 1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d⁶. Word that the 4s sublevel fills earlier than the 3d sublevel, a standard prevalence.
Exceptions to the Aufbau Precept: A Nearer Look
Whereas the Aufbau precept gives a superb basic guideline, there are exceptions. These come up as a result of delicate power stage interactions, particularly in transition metals and another components. For instance, chromium (Cr) and copper (Cu) have uncommon configurations as a result of stability gained by having a half-filled or fully stuffed d sublevel. These exceptions spotlight the complexity of electron interactions inside atoms.
The Significance of Electron Configuration in Chemistry
The electron configuration chart shouldn’t be merely a theoretical assemble; it has profound sensible implications in chemistry:
-
Predicting Chemical Properties: The association of electrons, significantly the valence electrons (outermost electrons), dictates how a component will react chemically. Parts with comparable valence electron configurations exhibit comparable chemical habits.
-
Understanding Bonding: The formation of chemical bonds (ionic, covalent, metallic) is determined by the electron configurations of the atoms concerned. Atoms have a tendency to realize, lose, or share electrons to attain a steady electron configuration, typically resembling that of a noble gasoline.
-
Spectroscopy: Electron configurations clarify the attribute spectral strains emitted or absorbed by atoms when electrons transition between power ranges. This kinds the premise of spectroscopic evaluation, a strong software in figuring out components and figuring out their composition.
-
Supplies Science: Understanding electron configurations is essential in designing and growing new supplies with particular properties. By manipulating the electron configuration of atoms, scientists can tailor supplies for numerous purposes, similar to semiconductors, superconductors, and catalysts.
Past the Fundamentals: Superior Ideas
Whereas the usual electron configuration chart gives a worthwhile overview, extra superior fashions exist to explain electron habits extra precisely. These embody:
-
Orbital Diagrams: These diagrams present the person orbitals inside every sublevel and the association of electrons inside them, together with their spin.
-
Quantum Mechanical Fashions: These refined fashions use quantum mechanics to explain the habits of electrons in atoms with higher precision, accounting for electron-electron interactions and relativistic results.
Conclusion: A Highly effective Instrument for Understanding Matter
The electron configuration chart is an indispensable software for understanding the elemental properties of components and their chemical habits. Its rules, whereas seemingly easy, underpin an unlimited quantity of chemical information. By understanding electron configurations, we acquire perception into the intricate workings of the atom and its function in shaping the macroscopic world round us. From predicting chemical reactions to designing new supplies, the electron configuration chart serves as a cornerstone of recent chemistry and associated fields. Its continued research stays important for developments in science and expertise.





Closure
Thus, we hope this text has offered worthwhile insights into Decoding the Universe: A Deep Dive into the Electron Configuration Chart of the Parts. We thanks for taking the time to learn this text. See you in our subsequent article!