Is Matter Round Us Pure? A Complete Exploration for Class 9
Associated Articles: Is Matter Round Us Pure? A Complete Exploration for Class 9
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Is Matter Round Us Pure? A Complete Exploration for Class 9. Let’s weave fascinating info and supply recent views to the readers.
Desk of Content material
Is Matter Round Us Pure? A Complete Exploration for Class 9
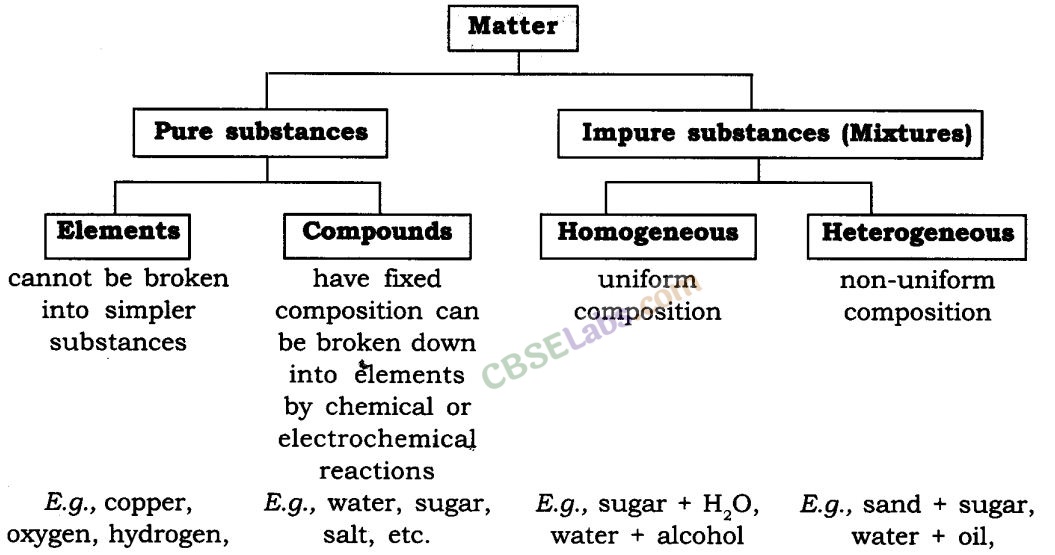
Matter, the bodily substance that makes up every little thing within the universe, exists in numerous varieties and states. Understanding the purity of this matter is essential in chemistry and varieties the idea for a lot of scientific explorations. This text delves into the idea of pure substances and mixtures, exploring their properties and classifications, culminating in an in depth flowchart summarizing the important thing distinctions.
What’s a Pure Substance?
A pure substance is a type of matter that has a continuing chemical composition and distinct properties. Which means its constituents are all equivalent on the molecular stage. It can’t be separated into totally different substances by bodily strategies. Pure substances could be additional divided into components and compounds.
-
Parts: These are the only types of matter. They can’t be damaged down into less complicated substances by chemical means. Every ingredient is characterised by its distinctive atomic quantity, representing the variety of protons in its nucleus. Examples embrace oxygen (O), hydrogen (H), iron (Fe), and gold (Au). Parts are represented by chemical symbols, a one or two-letter abbreviation primarily based on their identify (usually Latin).
-
Compounds: Compounds are fashioned when two or extra components chemically mix in a hard and fast ratio. This mix leads to a brand new substance with properties solely totally different from its constituent components. For instance, water (H₂O) is a compound fashioned from the mix of hydrogen and oxygen. The properties of water – its liquid state at room temperature, its means to dissolve many substances – are vastly totally different from the properties of hydrogen (a extremely flammable gasoline) and oxygen (a gasoline important for respiration). Compounds could be damaged down into their constituent components by way of chemical processes, equivalent to electrolysis.
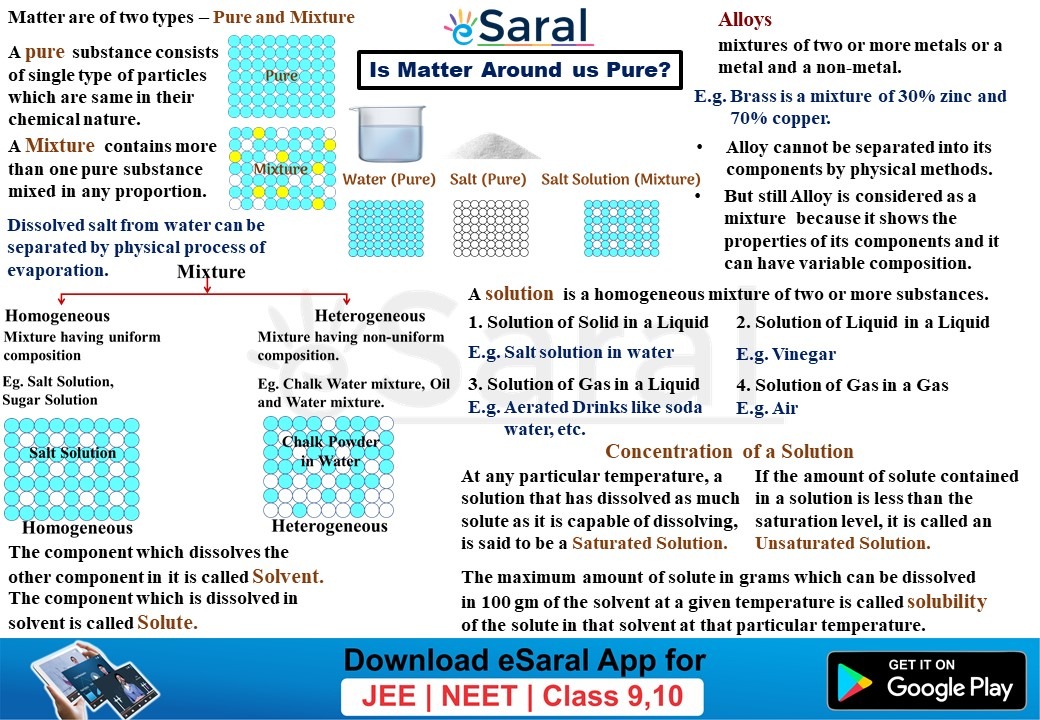
What’s a Combination?
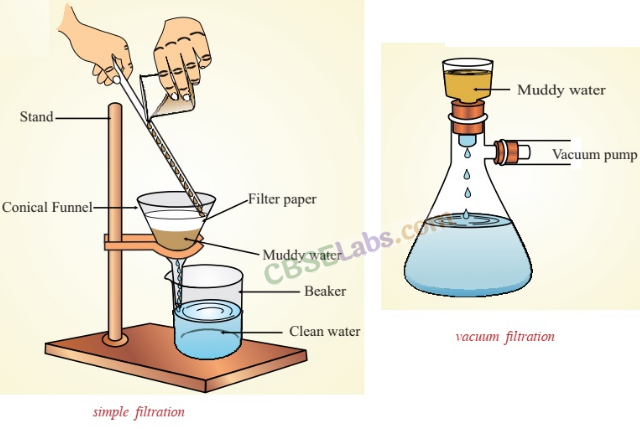
A combination is a mixture of two or extra substances that aren’t chemically bonded. The substances retain their particular person properties throughout the combination, and the composition shouldn’t be mounted. Mixtures could be separated into their parts by bodily strategies, equivalent to filtration, distillation, evaporation, or chromatography.
Mixtures are broadly labeled into two sorts:
-
Homogeneous Mixtures: These mixtures have a uniform composition all through. Which means the totally different parts are evenly distributed and indistinguishable to the bare eye. Examples embrace saltwater (salt dissolved in water), air (a combination of assorted gases), and sugar dissolved in water. In a homogeneous combination, the properties are constant all through the pattern.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition. The totally different parts are visibly distinguishable, and their distribution shouldn’t be uniform. Examples embrace sand and water, oil and water, and a salad. In a heterogeneous combination, totally different components of the pattern could have totally different properties.
Distinguishing between Pure Substances and Mixtures:
The important thing distinction between pure substances and mixtures lies of their composition and the strategies required for separation.
| Function | Pure Substance | Combination |
|---|---|---|
| Composition | Fastened and particular | Variable and indefinite |
| Separation | Can’t be separated by bodily strategies | Could be separated by bodily strategies |
| Properties | Fixed properties all through the pattern | Properties fluctuate all through the pattern (heterogeneous) or are uniform (homogeneous) |
| Melting/Boiling Level | Sharp, well-defined melting and boiling factors | Melting and boiling factors are over a spread |
| Parts | Just one sort of particle (ingredient or compound) | Two or extra kinds of particles |
Examples of Matter Round Us:
Let’s analyze the purity of some frequent substances:
-
Faucet Water: It is a combination. It incorporates water (H₂O) together with dissolved minerals, gases, and probably pollution. It may be purified by way of processes like distillation to acquire comparatively pure water.
-
Seawater: It is a heterogeneous combination. It incorporates water, dissolved salts (primarily sodium chloride), and numerous different substances like minerals and natural matter.
-
Air: It is a homogeneous combination of gases, primarily nitrogen, oxygen, argon, and carbon dioxide.
-
Milk: It is a heterogeneous combination containing water, fat, proteins, and sugars.
-
Gold: This is a component. Pure gold is a single ingredient with the image Au. Nonetheless, jewellery usually incorporates alloys (mixtures of gold with different metals).
-
Sugar: Pure desk sugar (sucrose) is a compound, a mixture of carbon, hydrogen, and oxygen atoms.
The Significance of Purity:
The purity of gear is essential in numerous fields. In drugs, pure substances are important for drug manufacturing and remedy. In business, the purity of supplies impacts the standard and efficiency of merchandise. In scientific analysis, pure substances are needed for correct and dependable experiments.
Flowchart Summarizing the Classification of Matter:
The next flowchart gives a visible illustration of the classification of matter, primarily based on its purity:
Matter
|
-----------------------------------------
| |
Pure Substance Combination
| |
--------------------- -------------------------
| | | |
Component Compound Homogeneous Combination Heterogeneous Combination
| | | |
(e.g., Gold, Oxygen) (e.g., Water, Salt) (e.g., Air, Saltwater) (e.g., Sand and Water, Salad)
Conclusion:
Understanding the excellence between pure substances and mixtures is key to comprehending the character of matter. The purity of a substance considerably impacts its properties and purposes. This text, together with the detailed flowchart, gives a complete overview of this important idea for Class 9 college students, enabling a deeper understanding of the world round them. Additional exploration into the strategies of separating mixtures and the detailed chemical properties of components and compounds will enrich this foundational information.






Closure
Thus, we hope this text has offered invaluable insights into Is Matter Round Us Pure? A Complete Exploration for Class 9. We thanks for taking the time to learn this text. See you in our subsequent article!