Understanding and Using Solubility Charts as per the Indian Pharmacopoeia (IP)
Associated Articles: Understanding and Using Solubility Charts as per the Indian Pharmacopoeia (IP)
Introduction
With enthusiasm, let’s navigate via the intriguing matter associated to Understanding and Using Solubility Charts as per the Indian Pharmacopoeia (IP). Let’s weave attention-grabbing data and supply contemporary views to the readers.
Desk of Content material
Understanding and Using Solubility Charts as per the Indian Pharmacopoeia (IP)

The Indian Pharmacopoeia (IP) is a vital doc for making certain the standard, security, and efficacy of pharmaceutical substances and preparations in India. A key side of pharmaceutical improvement and high quality management entails understanding the solubility of lively pharmaceutical components (APIs) and excipients. Solubility, outlined as the utmost quantity of a solute that may dissolve in a given quantity of solvent at a selected temperature and strain, is a basic physicochemical property instantly influencing varied points of drug formulation, together with bioavailability, stability, and manufacturing processes. The IP does not present a single, complete solubility chart, however relatively incorporates solubility data inside particular person monographs and basic chapters, providing helpful steering on decoding and making use of solubility information. This text will delve into the importance of solubility data throughout the IP, the other ways it is offered, and its sensible functions in pharmaceutical improvement and high quality management.
Solubility Terminology and Classification within the IP:
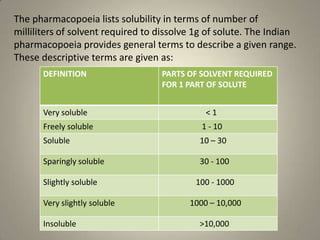
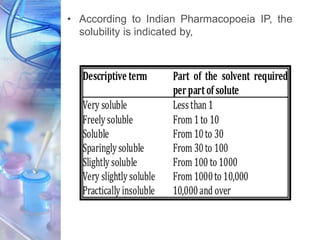
The IP employs a descriptive system to categorise the solubility of gear, sometimes expressed qualitatively relatively than quantitatively as actual numerical values. This qualitative description is commonly enough for a lot of pharmaceutical functions, offering a basic understanding of a substance’s solubility traits. The descriptive phrases used typically observe a scale much like that present in different pharmacopoeias, reminiscent of america Pharmacopeia (USP), though slight variations could exist. These phrases normally embody:
- Very soluble: Dissolves in lower than 1 a part of solvent.
- Freely soluble: Dissolves in 1 to 10 elements of solvent.
- Soluble: Dissolves in 10 to 30 elements of solvent.
- Sparingly soluble: Dissolves in 30 to 100 elements of solvent.
- Barely soluble: Dissolves in 100 to 1000 elements of solvent.
- Very barely soluble: Dissolves in 1000 to 10,000 elements of solvent.
- Virtually insoluble or insoluble: Dissolves in additional than 10,000 elements of solvent.
These phrases present a handy and readily comprehensible option to assess solubility. Nevertheless, it is essential to keep in mind that these are approximate values, and the precise solubility may be influenced by elements reminiscent of temperature, pH, the presence of different substances (co-solvents, surfactants), and the purity of the solvent. The IP monographs typically specify the solvent used for the solubility dedication, normally water, except in any other case acknowledged.
Finding Solubility Data within the IP:
Solubility data is not offered in a single, centralized desk throughout the IP. As a substitute, it is built-in into particular person monographs for every drug substance. Every monograph incorporates a bit devoted to the physicochemical properties of the drug, together with its solubility traits. This data is essential for choosing acceptable solvents and excipients throughout formulation improvement and making certain the drug’s dissolution and absorption within the physique. The IP additionally consists of basic chapters that present extra detailed steering on solubility dedication strategies and the interpretation of solubility information. These chapters would possibly talk about varied strategies, such because the shake-flask methodology or the saturation solubility methodology, providing a extra in-depth understanding of the method.
Significance of Solubility in Pharmaceutical Improvement:
Solubility performs a pivotal position in varied levels of drug improvement and manufacturing:
-
Formulation Improvement: The solubility of an API instantly impacts the selection of dosage kind. Extremely soluble medicine may be formulated into varied dosage kinds, whereas poorly soluble medicine typically require specialised formulations, reminiscent of stable dispersions, microemulsions, or nanoparticles, to reinforce their dissolution and bioavailability. The collection of excipients, reminiscent of solubilizers, co-solvents, or surfactants, can be guided by the API’s solubility traits.
-
Bioavailability: For a drug to exert its therapeutic impact, it should first dissolve within the organic fluids on the web site of absorption (e.g., gastrointestinal tract). Poorly soluble medicine typically exhibit low bioavailability as a result of they fail to dissolve adequately, limiting the quantity of drug obtainable for absorption. Understanding solubility is essential for predicting and enhancing drug bioavailability.
-
Stability: Solubility can affect the steadiness of a drug formulation. Adjustments in solubility, reminiscent of precipitation or crystallization, can result in instability and lowered shelf life. Cautious consideration of solubility is important for formulating steady and long-lasting drug merchandise.
-
Manufacturing Processes: Solubility impacts varied manufacturing processes, reminiscent of crystallization, drying, and granulation. Understanding the solubility habits of the API and excipients is essential for optimizing these processes and making certain the standard of the ultimate product.
-
High quality Management: Solubility testing is an integral a part of high quality management procedures. It ensures that the drug substance and the ultimate product meet the required specs. Discrepancies in solubility can point out points with the purity or high quality of the drug.
Past Qualitative Descriptions: Quantitative Solubility Knowledge:
Whereas the IP primarily makes use of qualitative solubility descriptions, quantitative solubility information is commonly crucial for extra exact formulation improvement and course of optimization. Researchers and producers typically make use of varied analytical strategies, reminiscent of UV-Vis spectrophotometry, HPLC, or dissolution testing, to find out the precise solubility of an API in numerous solvents and underneath various situations. This quantitative information permits for extra correct predictions of drug habits and allows the design of extra environment friendly and efficient formulations.
Conclusion:
Solubility is a important physicochemical property that considerably impacts the event, manufacturing, and efficacy of pharmaceutical merchandise. Whereas the IP does not present a single, complete solubility chart, it affords helpful qualitative solubility descriptions inside particular person drug monographs. This data, coupled with the understanding of related basic chapters and the appliance of quantitative solubility dedication strategies, is essential for pharmaceutical scientists and producers to design secure, efficient, and high-quality medicines. The qualitative information offered serves as a place to begin, guiding additional investigations and the appliance of extra exact quantitative strategies the place crucial. Steady understanding and utility of solubility ideas, as guided by the IP and supplemented by superior analytical strategies, stay important for developments in pharmaceutical science and the supply of efficient therapies.




Closure
Thus, we hope this text has offered helpful insights into Understanding and Using Solubility Charts as per the Indian Pharmacopoeia (IP). We recognize your consideration to our article. See you in our subsequent article!