Understanding the Constructing Blocks of Matter: A Deep Dive into Components and Valency
Associated Articles: Understanding the Constructing Blocks of Matter: A Deep Dive into Components and Valency
Introduction
With enthusiasm, let’s navigate via the intriguing matter associated to Understanding the Constructing Blocks of Matter: A Deep Dive into Components and Valency. Let’s weave fascinating info and provide contemporary views to the readers.
Desk of Content material
Understanding the Constructing Blocks of Matter: A Deep Dive into Components and Valency

The world round us, from the air we breathe to the bottom we stroll on, consists of matter. On the most elementary degree, this matter is constructed from parts – distinctive substances that can not be damaged down into easier substances by chemical means. These parts, organized within the periodic desk, exhibit an important property influencing their chemical conduct: valency. This text explores the character of parts, the periodic desk’s group, and the idea of valency, offering a complete understanding of how these ideas intertwine to manipulate chemical reactions and the formation of compounds.
Components: The Elementary Constructing Blocks
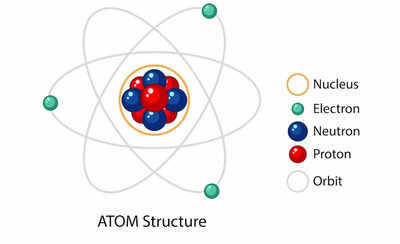
A component is outlined by the variety of protons in its nucleus, a amount often called its atomic quantity. Every aspect has a novel atomic quantity and, consequently, a novel set of chemical and bodily properties. These properties decide how a component interacts with different parts, forming molecules and compounds. For example, oxygen (atomic quantity 8) is a fuel important for respiration, whereas iron (atomic quantity 26) is a stable steel essential for building and organic processes. The range of parts and their distinctive properties are answerable for the unbelievable number of substances discovered within the universe.
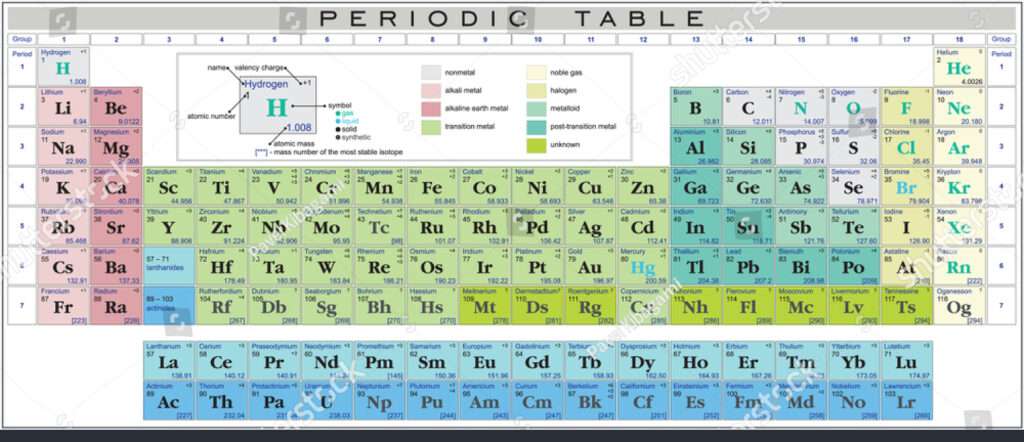
The periodic desk, a cornerstone of chemistry, organizes parts based mostly on their atomic quantity and recurring chemical properties. Components are organized in rows (durations) and columns (teams) reflecting their digital configurations and, consequently, their valency. The desk offers a concise abstract of the properties of all recognized parts, permitting scientists to foretell their conduct and interactions.
The Periodic Desk: A Structured Group of Components
The periodic desk’s association isn’t arbitrary. It displays the underlying quantum mechanical construction of atoms. As we transfer throughout a interval, the variety of electrons within the outermost shell (valence shell) will increase. This enhance in valence electrons immediately impacts a component’s reactivity and valency. As we transfer down a bunch, the variety of electron shells will increase, resulting in adjustments in atomic measurement and reactivity, however parts throughout the similar group share related valence electron configurations and thus related chemical properties.
The periodic desk is split into a number of classes based mostly on the properties of the weather:
- Alkali Metals (Group 1): Extremely reactive metals with one valence electron. They readily lose this electron to kind +1 ions.
- Alkaline Earth Metals (Group 2): Reactive metals with two valence electrons, forming +2 ions.
- Transition Metals (Teams 3-12): A various group of metals with variable valency, usually exhibiting a number of oxidation states.
- Halogens (Group 17): Extremely reactive nonmetals with seven valence electrons. They readily acquire one electron to kind -1 ions.
- Noble Gases (Group 18): Inert gases with a full valence shell (eight electrons, apart from helium with two). Their stability makes them unreactive.
- Metalloids (alongside the staircase): Components exhibiting properties of each metals and nonmetals, with variable valency.
- Lanthanides and Actinides: Two collection of parts positioned individually on the backside of the desk, recognized for his or her related chemical properties and complicated digital configurations.
Valency: The Driving Pressure Behind Chemical Bonding
Valency is a measure of a component’s combining capability – its capability to kind chemical bonds with different atoms. It represents the variety of electrons an atom can acquire, lose, or share to attain a secure electron configuration, usually resembling that of a noble fuel (a full octet of electrons within the valence shell). This drive in direction of stability is the basic driving pressure behind chemical bonding.
Components obtain stability via a number of kinds of chemical bonds:
-
Ionic Bonds: Fashioned via the electrostatic attraction between oppositely charged ions. This happens when one atom loses electrons (changing into a positively charged cation) and one other atom good points these electrons (changing into a negatively charged anion). The valency in ionic bonds is the same as the cost of the ion. For instance, sodium (Na) has a valency of +1, whereas chlorine (Cl) has a valency of -1, forming NaCl (sodium chloride).
-
Covalent Bonds: Fashioned by the sharing of electrons between atoms. This kind of bond is widespread between nonmetals. The valency in covalent bonds represents the variety of covalent bonds an atom can kind. For instance, carbon (C) has a valency of 4, forming 4 covalent bonds in methane (CH₄).
-
Metallic Bonds: Fashioned by the delocalized sharing of electrons amongst a lattice of steel atoms. This accounts for the attribute properties of metals, similar to conductivity and malleability. The valency in metallic bonds is much less simple and is usually associated to the variety of valence electrons contributed to the electron sea.
Valency Chart and its Purposes
A valency chart is a desk itemizing parts and their widespread valencies. It’s a priceless device for predicting the formulation of chemical compounds. Whereas some parts have a hard and fast valency, others exhibit variable valency, that means they will kind bonds with totally different numbers of electrons relying on the response situations and the opposite parts concerned. For example, iron (Fe) can exhibit valencies of +2 and +3, resulting in the formation of compounds like FeO (iron(II) oxide) and Fe₂O₃ (iron(III) oxide).
The valency chart helps in:
-
Predicting chemical formulation: Realizing the valencies of the constituent parts permits us to foretell the system of a compound. For instance, realizing that calcium (Ca) has a valency of +2 and chlorine (Cl) has a valency of -1, we will predict the system of calcium chloride as CaCl₂.
-
Balancing chemical equations: Valency performs an important function in balancing chemical equations, making certain that the variety of atoms of every aspect is conserved all through the response.
-
Understanding chemical reactions: Understanding the valency of parts helps us perceive why sure reactions happen and others don’t. Reactions are likely to proceed in a means that results in the formation of secure compounds with full valence shells.
-
Predicting the properties of compounds: The valency of parts influences the properties of the compounds they kind. For instance, compounds fashioned by parts with excessive valency usually have increased melting and boiling factors than these fashioned by parts with low valency.
Exceptions and Complexities
Whereas the idea of valency offers a helpful framework for understanding chemical bonding, there are exceptions and complexities. Transition metals, for instance, usually exhibit variable valency because of the involvement of d-electrons in bonding. Moreover, the idea of oxidation state, which represents the obvious cost of an atom in a compound, offers a extra nuanced description of the electron distribution in some circumstances, notably for compounds with covalent bonds.
Conclusion
Components and valency are elementary ideas in chemistry. The periodic desk’s group displays the underlying digital construction of atoms, immediately influencing their valency and reactivity. Understanding valency is essential for predicting chemical formulation, balancing equations, and comprehending the conduct of chemical reactions. Whereas the idea simplifies the complicated world of chemical bonding, it offers a robust device for understanding and predicting the conduct of matter, forming the muse for additional exploration in numerous branches of chemistry and associated fields. Additional research into superior ideas like oxidation states and coordination chemistry will present a deeper understanding of the complexities past the fundamental valency mannequin. Nevertheless, a stable grasp of elemental properties and valency stays important for any scholar or skilled working within the discipline of chemistry.





Closure
Thus, we hope this text has offered priceless insights into Understanding the Constructing Blocks of Matter: A Deep Dive into Components and Valency. We thanks for taking the time to learn this text. See you in our subsequent article!