Understanding the Interaction of pH and ORP: A Complete Information with Chart Interpretation
Associated Articles: Understanding the Interaction of pH and ORP: A Complete Information with Chart Interpretation
Introduction
With enthusiasm, let’s navigate via the intriguing subject associated to Understanding the Interaction of pH and ORP: A Complete Information with Chart Interpretation. Let’s weave attention-grabbing data and provide recent views to the readers.
Desk of Content material
Understanding the Interaction of pH and ORP: A Complete Information with Chart Interpretation
Water high quality is paramount in numerous functions, from aquaculture and hydroponics to industrial processes and ingesting water therapy. Two essential parameters defining water high quality are pH (potential of hydrogen) and ORP (oxidation-reduction potential). Whereas distinct, these parameters are intricately linked, influencing one another and impacting the general chemical surroundings. This text delves into the person traits of pH and ORP, explores their relationship, and supplies a complete information to deciphering a mixed pH vs. ORP chart.
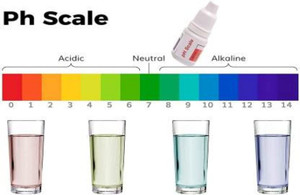
pH: A Measure of Acidity and Alkalinity
pH is a logarithmic scale starting from 0 to 14, representing the focus of hydrogen ions (H+) in an answer. A pH of seven is taken into account impartial, indicating an equal focus of H+ and hydroxide ions (OH-). Values under 7 are acidic, with decrease values indicating increased acidity, whereas values above 7 are alkaline (or primary), with increased values indicating higher alkalinity. Every complete quantity change on the pH scale represents a tenfold change in H+ focus. As an illustration, an answer with a pH of 4 is ten occasions extra acidic than an answer with a pH of 5.
The pH of water considerably impacts numerous chemical and organic processes. In aquatic programs, pH impacts the solubility of vitamins, the toxicity of heavy metals, and the survival and progress of aquatic organisms. For instance, many fish species have a slender pH tolerance vary, and deviations outdoors this vary can result in stress, illness, and mortality. In industrial processes, pH management is essential for sustaining the effectivity and effectiveness of chemical reactions.
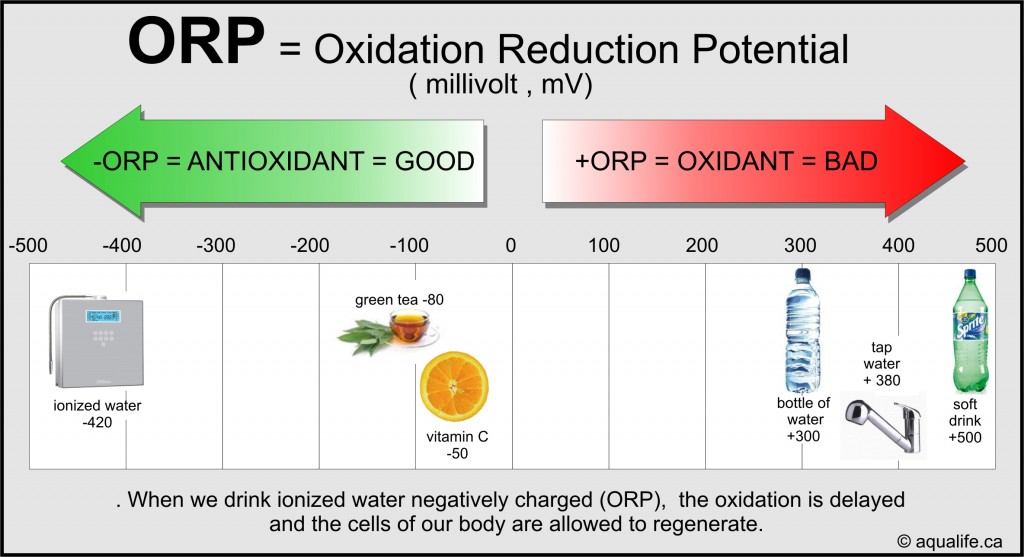
ORP: A Measure of Oxidizing and Lowering Capability
ORP, often known as redox potential, measures the tendency of an answer to realize or lose electrons. It’s expressed in millivolts (mV). A constructive ORP worth signifies an oxidizing surroundings, the place substances readily lose electrons (are oxidized). A unfavourable ORP worth signifies a lowering surroundings, the place substances readily achieve electrons (are diminished). The upper the constructive ORP worth, the stronger the oxidizing energy, and vice versa for unfavourable values.
ORP is an important indicator of the presence and exercise of oxidizing and lowering brokers in an answer. Oxidizing brokers, resembling chlorine and ozone, are generally used for disinfection and water therapy. Lowering brokers, like sulfides and ferrous iron, can contribute to undesirable odors, tastes, and corrosion. In organic programs, ORP performs a major function in metabolic processes, influencing microbial exercise and the bioavailability of vitamins. For instance, a excessive ORP surroundings can inhibit the expansion of anaerobic micro organism, whereas a low ORP surroundings can promote their progress.
The Interaction of pH and ORP
pH and ORP are usually not unbiased parameters; they work together and affect one another. The pH of an answer can have an effect on its ORP, and adjustments in ORP can, in flip, have an effect on the pH. This interplay is advanced and depends upon a number of elements, together with the precise chemical composition of the answer, temperature, and the presence of redox {couples} (pairs of oxidized and diminished species).
As an illustration, the presence of dissolved oxygen (O2) considerably influences each pH and ORP. Oxygen acts as an oxidizing agent, growing the ORP. Moreover, the discount of oxygen can result in the formation of hydroxide ions (OH-), growing the pH. Conversely, the presence of lowering brokers like sulfides can lower the ORP and doubtlessly decrease the pH.
Decoding a pH vs. ORP Chart
A mixed pH vs. ORP chart supplies a visible illustration of the connection between these two parameters in a given resolution. The chart usually plots pH on the x-axis and ORP on the y-axis. Every level on the chart represents a particular mixture of pH and ORP values. By analyzing the distribution of factors, one can achieve worthwhile insights into the general chemical surroundings.
Decoding Key Options:
-
Clustering of Factors: A cluster of factors signifies a comparatively secure chemical surroundings with constant pH and ORP values. This stability may be indicative of a well-buffered system or a system that’s not present process vital chemical adjustments.
-
Scattered Factors: Scattered factors counsel a fluctuating chemical surroundings with variations in each pH and ORP. This variability may point out instability, ongoing chemical reactions, or exterior influences.
-
Developments and Correlations: Analyzing tendencies within the knowledge can reveal correlations between pH and ORP. A constructive correlation means that as pH will increase, ORP additionally tends to extend, whereas a unfavourable correlation suggests an inverse relationship. Understanding these correlations may help establish the dominant chemical processes occurring within the system.
-
Reference Traces: Some pH vs. ORP charts embrace reference traces representing particular chemical situations or thresholds. These traces may help establish potential issues or areas of concern. For instance, a line may point out the ORP required for efficient disinfection or the pH vary appropriate for a selected aquatic organism.
Functions of pH vs. ORP Charts:
pH vs. ORP charts discover functions in numerous fields, together with:
-
Water Remedy: Monitoring pH and ORP throughout water therapy processes helps guarantee efficient disinfection, removing of contaminants, and upkeep of optimum water high quality.
-
Aquaculture: Monitoring pH and ORP in aquaculture programs helps keep a wholesome surroundings for fish and different aquatic organisms. Deviations from optimum ranges can point out potential issues, resembling algal blooms or bacterial infections.
-
Hydroponics: Exact management of pH and ORP is essential in hydroponic programs to make sure optimum nutrient availability and plant progress.
-
Industrial Processes: Monitoring pH and ORP in industrial processes is important for controlling chemical reactions, stopping corrosion, and making certain product high quality.
-
Environmental Monitoring: pH and ORP measurements are used to evaluate the standard of pure water our bodies and establish potential air pollution sources.
Limitations and Issues:
Whereas pH vs. ORP charts present worthwhile data, it is essential to acknowledge their limitations:
-
Simplified Illustration: The charts present a simplified illustration of a fancy chemical system. They don’t seize the complete spectrum of chemical interactions and processes occurring within the resolution.
-
Context is Essential: The interpretation of pH vs. ORP knowledge should all the time be thought of throughout the particular context of the system being monitored. The optimum ranges for pH and ORP range extensively relying on the applying.
-
Different Parameters: pH and ORP are solely two of many parameters that affect water high quality. A complete evaluation requires contemplating different elements, resembling temperature, dissolved oxygen, conductivity, and the presence of particular contaminants.
Conclusion:
pH and ORP are important parameters for assessing and controlling water high quality throughout various functions. Understanding their particular person traits and their intricate interaction is essential for efficient administration and optimization of varied programs. A mixed pH vs. ORP chart supplies a robust instrument for visualizing this relationship and gaining worthwhile insights into the chemical surroundings. Nonetheless, deciphering these charts requires cautious consideration of the precise context and limitations of the information. By combining chart evaluation with different related parameters and professional information, a complete understanding of water high quality may be achieved, resulting in improved effectivity and sustainability in numerous sectors.






Closure
Thus, we hope this text has offered worthwhile insights into Understanding the Interaction of pH and ORP: A Complete Information with Chart Interpretation. We thanks for taking the time to learn this text. See you in our subsequent article!
