Understanding the Relationship Between pH and Alkalinity: A Complete Information with Conversion Charts
Associated Articles: Understanding the Relationship Between pH and Alkalinity: A Complete Information with Conversion Charts
Introduction
With enthusiasm, let’s navigate via the intriguing subject associated to Understanding the Relationship Between pH and Alkalinity: A Complete Information with Conversion Charts. Let’s weave attention-grabbing info and supply recent views to the readers.
Desk of Content material
Understanding the Relationship Between pH and Alkalinity: A Complete Information with Conversion Charts
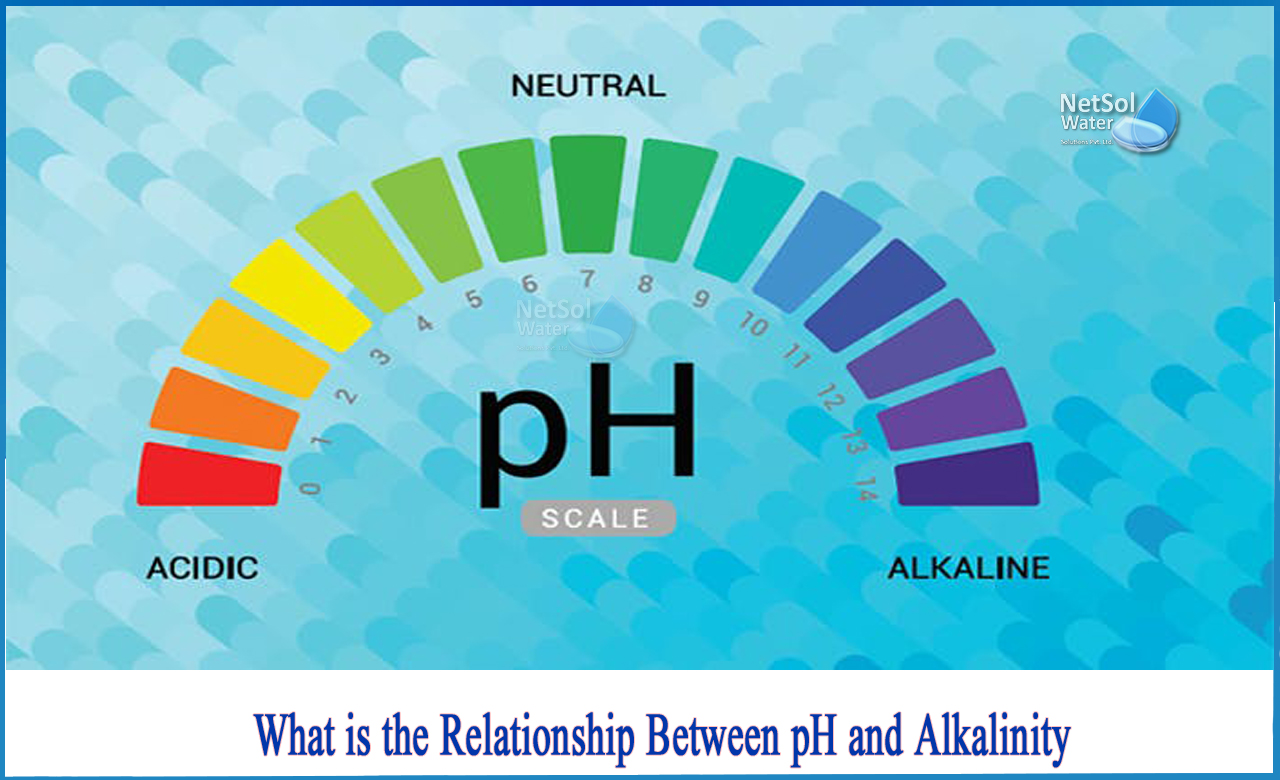
The phrases pH and alkalinity are incessantly utilized in numerous fields, together with water remedy, aquaculture, and soil science. Whereas seemingly associated, they symbolize distinct but interconnected water high quality parameters. Understanding their variations and the connection between them is essential for efficient administration and management in quite a few purposes. This text delves into the complexities of pH and alkalinity, explains their interrelationship, and gives an in depth information to decoding and using pH to alkalinity conversion charts.
pH: A Measure of Hydrogen Ion Focus
pH is a logarithmic scale that measures the focus of hydrogen ions (H⁺) in an answer. It ranges from 0 to 14, with 7 representing neutrality. A pH under 7 signifies acidity (larger H⁺ focus), whereas a pH above 7 signifies alkalinity (decrease H⁺ focus). Every complete quantity change on the pH scale represents a tenfold change in hydrogen ion focus. For instance, an answer with a pH of 6 is ten instances extra acidic than an answer with a pH of seven.
The pH of an answer considerably impacts numerous chemical and organic processes. For example, in aquatic methods, pH impacts the solubility of vitamins, the toxicity of heavy metals, and the survival of aquatic organisms. In soil, pH influences nutrient availability and microbial exercise.
Alkalinity: A Measure of Buffering Capability
Alkalinity, not like pH, is a measure of a water physique’s capability to withstand adjustments in pH. It represents the whole focus of bases within the water that may neutralize acids. These bases primarily embrace bicarbonate (HCO₃⁻), carbonate (CO₃²⁻), and hydroxide (OH⁻) ions. Alkalinity is often expressed in milligrams per liter (mg/L) as calcium carbonate (CaCO₃).
Excessive alkalinity signifies a water physique’s means to soak up vital quantities of acid with out experiencing a drastic pH drop. This buffering capability is essential for sustaining a steady pH, defending aquatic life from sudden pH fluctuations, and stopping harm to infrastructure in water remedy methods. Conversely, low alkalinity signifies a susceptible system inclined to speedy pH adjustments, probably resulting in antagonistic penalties.
The Interaction Between pH and Alkalinity
Whereas pH and alkalinity are distinct measurements, they’re intimately linked. The connection is advanced and never simply represented by a single, universally relevant conversion chart. It is because the alkalinity of an answer relies upon not solely on the focus of bases but in addition on the precise varieties of bases current and the pH of the answer. The carbonate system, involving the equilibrium between carbonic acid (H₂CO₃), bicarbonate (HCO₃⁻), and carbonate (CO₃²⁻) ions, performs a dominant function in figuring out each pH and alkalinity in pure waters.
The pH of an answer influences the relative proportions of bicarbonate, carbonate, and carbonic acid. At decrease pH values, bicarbonate is the dominant species, whereas at larger pH values, carbonate turns into extra prevalent. This equilibrium shift impacts the alkalinity worth, making a direct conversion unattainable with out realizing the precise chemical composition of the answer.
Limitations of pH to Alkalinity Conversion Charts
The shortage of a easy, common conversion method stems from the complexity of the carbonate system and the presence of different bases that contribute to alkalinity. Any chart making an attempt to correlate pH and alkalinity should make assumptions in regards to the chemical composition of the water, which can not all the time be correct.
Subsequently, any pH to alkalinity conversion chart must be thought of an approximation, helpful solely underneath particular circumstances and with a transparent understanding of its limitations. Such charts are most dependable when utilized to waters dominated by the carbonate system, with minimal interference from different bases or acids.
Kinds of pH to Alkalinity Conversion Charts and Their Purposes
A number of varieties of charts try and symbolize the connection between pH and alkalinity. These charts typically current alkalinity as a operate of pH, assuming a selected chemical composition (e.g., predominantly bicarbonate alkalinity). They’re sometimes used as a fast estimation instrument moderately than a exact calculation methodology.
-
Simplified Charts: These charts present a basic overview of the connection, displaying approximate alkalinity ranges for various pH values. They’re helpful for preliminary assessments however lack the precision wanted for correct calculations.
-
Charts Primarily based on Particular Chemical Compositions: These charts are extra correct however require data of the water’s chemical make-up, particularly the relative proportions of bicarbonate, carbonate, and hydroxide ions. They will present extra dependable estimates throughout the specified circumstances.
-
Nomograms: Nomograms are graphical instruments that use a number of scales to facilitate calculations. Some nomograms are designed to estimate alkalinity primarily based on pH and different parameters, akin to whole dissolved solids (TDS) or temperature.
Utilizing pH and Alkalinity Information Collectively
As a substitute of relying solely on a conversion chart, it is extra informative and correct to make the most of each pH and alkalinity measurements collectively to evaluate water high quality. The mixed knowledge present a extra complete understanding of the water’s buffering capability and its potential to withstand pH adjustments.
For instance, a water pattern with a pH of seven and excessive alkalinity signifies a well-buffered system able to resisting pH shifts. Conversely, a water pattern with a pH of seven and low alkalinity suggests a system susceptible to pH fluctuations, probably resulting in instability.
Sensible Purposes and Examples
The mixed use of pH and alkalinity knowledge is essential in numerous purposes:
-
Aquaculture: Sustaining optimum pH and alkalinity ranges is crucial for the well being and productiveness of aquatic organisms. Common monitoring and changes are needed to forestall stress and illness.
-
Water Therapy: Understanding the connection between pH and alkalinity is significant for efficient water remedy processes, akin to coagulation, flocculation, and disinfection. Changes to pH and alkalinity are sometimes wanted to optimize these processes.
-
Soil Science: Soil pH and alkalinity considerably affect nutrient availability and plant progress. Adjusting soil pH and alkalinity via liming or different strategies can enhance soil fertility and crop yields.
-
Swimming Pool Administration: Correct pH and alkalinity management is essential for sustaining a secure and fulfilling swimming atmosphere. Imbalances can result in corrosion of pool tools and pores and skin irritation.
Conclusion
Whereas a direct conversion between pH and alkalinity is just not doable as a result of complexity of the chemical interactions concerned, understanding their interrelationship is crucial for efficient water high quality administration. Utilizing pH and alkalinity measurements collectively gives a much more full image than counting on approximate conversion charts. As a substitute of in search of a direct conversion, concentrate on understanding the interaction between these parameters to successfully handle and management water high quality in numerous purposes. Do not forget that any conversion chart must be used with warning, acknowledging its limitations and counting on skilled judgment and extra chemical evaluation for exact determinations. All the time seek the advice of related tips and requirements for particular purposes to make sure correct and dependable outcomes.






![Understanding pH and Alkalinity in the Pool: A Simplified Guide[ 2024]](https://thepoolanddeck.com/wp-content/uploads/2023/11/TPAD2024-006-1024x576.png)

Closure
Thus, we hope this text has supplied useful insights into Understanding the Relationship Between pH and Alkalinity: A Complete Information with Conversion Charts. We admire your consideration to our article. See you in our subsequent article!